valence electrons in aluminum|Valence Electrons Chart for All Elements : Tagatay Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. The number of valence electrons in one atom of each . After teasing of a possible comeback, Vlogger couple JaMill to YouTube on Wednesday, September 15. In a new Youtube video, couple Jayzam Manabat and Camille Trinidad, explained why they had to delete their channel with . Jayzam could be referring to a cheating scandal that Camille accused him early this year.
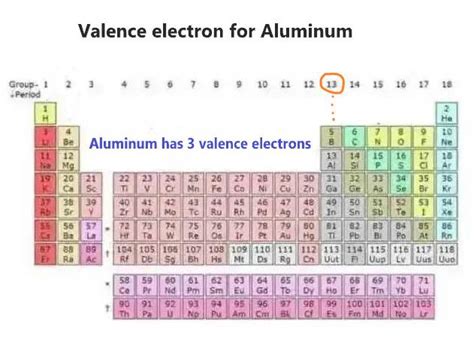
valence electrons in aluminum,You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups (columns) of the periodic table.
Mar 23, 2023
There are two ways to find the number of valence electrons in Aluminum (Al). The first is to use the Periodic Table to figure out how many electrons Aluminum.
Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. The number of valence electrons in one atom of each . Write the configuration of Aluminum, assuming that it has lost its valence electrons. What happens to aluminum when it reacts with chlorine? Balance these . Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the \(1s\) sublevel are called . Aluminum Valence Electrons: Aluminum is the chemical element denoted with a symbol (Al) and with an atomic number 13. Aluminum is in silvery-white color metal. Al is metal used as a nonferrous metal. Its .
For aluminum, Z=13...and there is a neon core of electrons... We describe the electronic configuration as 1s^(2)2s^(2)2p^(6)3s^(2)3p^1. The valence electrons all have . Elements from groups have preferred numbers of valence electrons. For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. Alkaline earth atoms (e.g., magnesium, calcium) have two .How can you use the periodic table to find out how many valence electrons an element has? This article from Khan Academy explains the simple rules and patterns that can help you determine the number of electrons in the outermost shell of any atom. You will also learn why valence electrons are important for chemical bonding and reactivity.What is the electron arrangement of aluminum? How many valence electrons does it have? Solution. Aluminum has 13 electrons so it will have the electron arrangement (2, 8, 3) which represents two electrons in the \(n=1\) energy level, eight electrons in the \(n=2\) level, and three electrons in the \(n=3\) level. Aluminum has three valence .valence electrons in aluminumThe electrons that determine valence – how an atom reacts chemically – are those with the highest energy.. For a main-group element, the valence electrons are defined as those electrons residing in the electronic shell of highest principal quantum number n. [1] Thus, the number of valence electrons that it may have depends on the electron configuration in a .
Answer: Aluminum has 3 valence electrons. Explanation: Valence electrons are the electrons present in the outermost energy level (shell) of an atom. Aluminum (Al), with an atomic number of 13, has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 1. In this configuration, the outermost shell is the third shell (3s 2 3p 1), which contains 3 .Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons . The electronic structure associated with chemical bonding affects all properties of materials except radioactivity. In metals, the widely taught concept of an ideal metal or free electron gas is popularly expressed as a regular array of metal ions surrounded by a sea of delocalized valence electrons ().Although a gross simplification, this model is often sufficient .
Hence, in this way we can calculate the number of valence electrons in aluminium. Note: As we know that valency is the combining power of an element. It is found that elements in the same group have the same valence in the periodic table. Basically the valence of an element is related to the electrons that are present in the outer shell.Valence Electrons Chart for All Elements The valence electrons are the electrons in the outermost electron shell of an atom.. That is why elements whose atoms have the same number of valence electrons are grouped together in the Periodic Table.. Generally, elements in Groups 1, 2, and 13 to 17 tend to react to form a closed shell, corresponding to the electron configuration #s^2p^6#.. This tendency is called the octet . There are two ways to find the number of valence electrons in Aluminum (Al). The first is to use the Periodic Table to figure out how many electrons Aluminum.
Accurate and high reciprocal resolution experimental structure factors of aluminum were determined from a synchrotron powder X-ray diffraction data measured at 30 K with sin θ/λ < 2.31 Å−1. This periodic table shows the valences of element groups. The transition metals make use of the d-subshell, which can accommodate 10 electrons.The f-subshell holds 14 electrons and the g-subshell contains up to .valence electrons in aluminum Valence Electrons Chart for All Elements Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant. Now, the electron configuration of aluminum shows that the last orbit has three electrons. Therefore, the valence electrons of aluminum are three. The last shell of aluminum has three unpaired electrons, so the .

For example, the covalent radius of an aluminum atom (1s 2 2s 2 2p 6 3s 2 3p 1) is 118 pm, whereas the ionic radius of an Al 3 + . For example, Sc and Ga both have three valence electrons, so the rapid increase in ionization energy occurs after the third ionization. Table \(\PageIndex{2}\): Successive Ionization Energies for Selected Elements . The valence electrons of each main-group element can be determined by the column in which it is located. (i.e., all group 1 elements have 1 valence electron, all group 2 elements have 2 valence electrons, skip the transition metals. then, all group 13 elements have 3 valence electrons, all group 14 elements have 4 valence electrons, and so on up to group . Khanmigo is now free for all US educators! Plan lessons, develop exit tickets, and so much more with our AI teaching assistant.
The electron configuration for Aluminum is. 1 s 2 2 s 2 2 P 6 6 3 s 2 3 P 1 The first 10 electrons are in the filled first and second shells. This leaves only three electrons in the third shell 3 s 2 3 p 1. 1 3 − 1 0 = 3. so there are only 3 valance electrons
A valence electron is an electron that is associated with an atom, and that can participate in the formation of a chemical bond; in a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. The presence of valence electrons can determine the element's chemical properties and whether it may .
valence electrons in aluminum|Valence Electrons Chart for All Elements
PH0 · What is the number of valence electrons in aluminum?
PH1 · What Are Valence Electrons? Definition and Periodic
PH2 · Valences of the Chemical Elements
PH3 · Valence Electrons Chart for All Elements
PH4 · How to Find Valence Electrons for Aluminum (Al)
PH5 · How Many Valence Electrons Does Aluminum (Al) Have?
PH6 · Chemistry of Aluminum (Z=13)
PH7 · Aluminum Valence Electrons
PH8 · 3.1: Valence Electrons
PH9 · 10.6: Valence Electrons